Check Your UK University Rank here
All comments, criticism, remarks etc are most welcome.
Faculty satisfaction with the ranking methods are very variable - Tell us more.
Tuesday, January 25, 2011
Wednesday, January 12, 2011
Engineering Courses in Ontario Canada options and degree courses in Metallurgy and Metallurgical Transformation
Let me give readers another perspective and highly successful approach to Engineering. Engineering Courses in Ontario Canada brought my attention to Conestoga Community College a most fruitful window on education and employment in Ontario, Canada.
Conestoga is a young University and Polytechnic with an enviable record:
-Since it's inauguration in 1967, it has been rated N°1 College in Ontario for nine of the last eleven years.
-Graduate job placement rate is 94.2% six months after graduation.
-Conestoga is an outstanding Polytechnical Institute and a founding member of Polytechnics Canada
-Full-time Students: 8,500 - Apprenticeship Students: 4,000 - Part-time Students: 38,000
-40% of all adults in Waterloo Region have taken at least one course at Conestoga.
-Conestoga graduates annually inject $1.4 billion into the local economy.
-It offers 120 full-time programs, 37 apprenticeship related programs, 7 four-year bachelor degree programs, 3 collaborative degrees, 25 post-graduate certificates and over 100 part-time programs.
-Conestoga has six campuses: Doon (Kitchener), Waterloo, Guelph, Stratford, Cambridge and Ingersoll.
-Conestoga’s loan default rate is the lowest in the province at 6.8%. We attribute this, at least partially, to the fact so many of our students get excellent jobs after graduation! Last year Conestoga provided $20 million in student loans.
AIMS
-To champion innovation and excellence in education and training.
-To serve responsibly the diverse and ever-changing needs of the community.
-To inspire students and employees to strive toward their highest potential.
METALLURGY and MECHANICAL PROCESS ENGINEERING
-Powder Metallurgy.
-Welding
-BSc in Applied Mechanical Systems-extrusion, wire and bar drawing, sheet metal working, powder metallurgy
-MSc and BSc degrees conferred[Pdf]
MORE on Conestoga :
-Introduction,
-Community-Colleges Index.htmls.net
-reports
Canadian Colleges and Cegeps – « Collège d'enseignement général et professionnel ».
Canada currently has 146 colleges and cegeps in 115 towns and cities covering 13 provinces and territories. We provide access to all the key information you need to know about each community college, technical college or cegep in Canada. You can browse colleges by city or province. Each college listing will provide information such as number of students, contact info, admissions, tuition fees, financial aid and interesting facts about the college. You can also access our college program directory if you are looking for information about a specific program or area of study.
More on education in Ontario
All Canada
Conestoga is a young University and Polytechnic with an enviable record:
-Since it's inauguration in 1967, it has been rated N°1 College in Ontario for nine of the last eleven years.
-Graduate job placement rate is 94.2% six months after graduation.
-Conestoga is an outstanding Polytechnical Institute and a founding member of Polytechnics Canada
-Full-time Students: 8,500 - Apprenticeship Students: 4,000 - Part-time Students: 38,000
-40% of all adults in Waterloo Region have taken at least one course at Conestoga.
-Conestoga graduates annually inject $1.4 billion into the local economy.
-It offers 120 full-time programs, 37 apprenticeship related programs, 7 four-year bachelor degree programs, 3 collaborative degrees, 25 post-graduate certificates and over 100 part-time programs.
-Conestoga has six campuses: Doon (Kitchener), Waterloo, Guelph, Stratford, Cambridge and Ingersoll.
-Conestoga’s loan default rate is the lowest in the province at 6.8%. We attribute this, at least partially, to the fact so many of our students get excellent jobs after graduation! Last year Conestoga provided $20 million in student loans.
AIMS
-To champion innovation and excellence in education and training.
-To serve responsibly the diverse and ever-changing needs of the community.
-To inspire students and employees to strive toward their highest potential.
METALLURGY and MECHANICAL PROCESS ENGINEERING
-Powder Metallurgy.
-Welding
-BSc in Applied Mechanical Systems-extrusion, wire and bar drawing, sheet metal working, powder metallurgy
-MSc and BSc degrees conferred[Pdf]
MORE on Conestoga :
-Introduction,
-Community-Colleges Index.htmls.net
-reports
Canadian Colleges and Cegeps – « Collège d'enseignement général et professionnel ».
Canada currently has 146 colleges and cegeps in 115 towns and cities covering 13 provinces and territories. We provide access to all the key information you need to know about each community college, technical college or cegep in Canada. You can browse colleges by city or province. Each college listing will provide information such as number of students, contact info, admissions, tuition fees, financial aid and interesting facts about the college. You can also access our college program directory if you are looking for information about a specific program or area of study.
More on education in Ontario
All Canada
Wednesday, December 29, 2010
Materials and Environment - New book entry and link for extensive online reviewing.
Well worth a mention:
Materials Engineering Science Processing and Design by Ashby,Shercliff & Cebon, renowned specialists in the field of Materials Selection and Environment, may be extensively reviewed with a view to acquisition at the link provided (scribd) I have added the link in my side bar entitled Materials and Environment.
For readers of this post a convenient link is Materials Engineering Science Processing and Design
Alternative links for extensive reviewing from Google Books
Amazon
Materials Engineering Science Processing and Design by Ashby,Shercliff & Cebon, renowned specialists in the field of Materials Selection and Environment, may be extensively reviewed with a view to acquisition at the link provided (scribd) I have added the link in my side bar entitled Materials and Environment.
For readers of this post a convenient link is Materials Engineering Science Processing and Design
Alternative links for extensive reviewing from Google Books
Amazon
Tuesday, December 28, 2010
Materials Science and Technology -Edited by Maney Press, Leeds,UK for IOM3
Materials Science and Technology Up-date:
This highly reputable peer reviewed journal is published monthly.
The journal covers fundamental and technological aspects of the properties, characterisation, modelling, processing, and fabrication of engineering materials, with a particular interest in microstructure–property–processing relationships. Now the full archives from its first issue on January 1985 to date are available online. At present all papers from 1st of January 1990 are freely available to members of The Institute of Materials Minerals and Mining: Logo below:
Meet the author via his seminal paper in Materials Science and Technology:
Optimizing deoxidation and desulphurization during vacuum induction melting of alloy 718
pp. 167-170(4) Author: Alexander, J.
This paper marked an important step in improved quality and cost of premium quality, superalloys for aeroengine hot parts. It was a first in qualifying recycled alloy for use in aeroengine for civil aircraft use. (in 1985 a military aeroengine was required to withstand 5000hrs in service, whereas aero engines in civilian -passenger aircraft were required to withstand 50 000hrs in service, a factor of 10, a full order of magnitude.
Should manufacturers, would be manufacturers or users require further information please do not hesitate to contact the author.
Other member benefits:
Members have full access to all papers, in over 20 peer reviewed journals, from 1990 on, either to peruse online or to download. Discover all the Institute supported Journals
There are of course also many added benefits for members indispensable for progressing within the profession: Chartered Professional Engineer, CEng, Eur Eng, Fellowships,Conferences, Meetings, specialised Careers Advice and Job Adverts and more. Please visit IOM3
Many Fellows are also Fellows of the Royal Society (FRS) and Fellows of Engineering (FEng)
Should you as a reader decide to become a member please let me know.
Thanks in advance and enjoy becoming involved in a profession so fundamental to any modern economy,
Books by members
This highly reputable peer reviewed journal is published monthly.
The journal covers fundamental and technological aspects of the properties, characterisation, modelling, processing, and fabrication of engineering materials, with a particular interest in microstructure–property–processing relationships. Now the full archives from its first issue on January 1985 to date are available online. At present all papers from 1st of January 1990 are freely available to members of The Institute of Materials Minerals and Mining: Logo below:
Meet the author via his seminal paper in Materials Science and Technology:
Optimizing deoxidation and desulphurization during vacuum induction melting of alloy 718
pp. 167-170(4) Author: Alexander, J.
This paper marked an important step in improved quality and cost of premium quality, superalloys for aeroengine hot parts. It was a first in qualifying recycled alloy for use in aeroengine for civil aircraft use. (in 1985 a military aeroengine was required to withstand 5000hrs in service, whereas aero engines in civilian -passenger aircraft were required to withstand 50 000hrs in service, a factor of 10, a full order of magnitude.
Should manufacturers, would be manufacturers or users require further information please do not hesitate to contact the author.
Other member benefits:
Members have full access to all papers, in over 20 peer reviewed journals, from 1990 on, either to peruse online or to download. Discover all the Institute supported Journals
There are of course also many added benefits for members indispensable for progressing within the profession: Chartered Professional Engineer, CEng, Eur Eng, Fellowships,Conferences, Meetings, specialised Careers Advice and Job Adverts and more. Please visit IOM3
Many Fellows are also Fellows of the Royal Society (FRS) and Fellows of Engineering (FEng)
Should you as a reader decide to become a member please let me know.
Thanks in advance and enjoy becoming involved in a profession so fundamental to any modern economy,
Books by members
Wednesday, November 17, 2010
Phase Diagrams for Metal and Alloy
The mechanical properties of materials depend strongly upon microstructure and the development of microstructure of an alloy is related to the characteristics of its phase diagram. Therefore, the understanding of phase diagrams for alloy systems is very important. Phase diagram is very useful to represent the most stable relationships between phases in alloy systems. In addition, phase diagrams provide valuable information about melting, casting, crystallization, and other phenomena.
Before interpret and utilize phase diagram, it is necessary to understand component, system and phase. Components are pure metals and/or compounds of which an alloy is composed. For example, in a copper–zinc brass, the components are Cu and Zn. System may refer to a specific body of material under consideration (e.g., a ladle of molten steel). Or it may relate to the series of possible alloys consisting of the same components, but without regard to alloy composition (e.g., the iron–carbon system). Phase may be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics. Every pure material is considered to be a phase; so also is every solid, liquid, and gaseous solution.
Unary System
Perhaps the simplest and easiest type of phase diagram to understand is that for a one-component system. In a one-component system, or unary system, however, the composition does not vary, but must always be unity. Therefore there are only two variables which can vary: pressure and temperature. Every possible combination of temperature and pressure can be readily represented by points on a two-dimensional diagram. Three phases (solid-, liquid, and vapor-phase) are found on this type of phase diagram.
Binary System
Another type of common phase diagram is that for two components or binary system. Binary phase diagrams represent the relationships between temperature and the compositions of phases at equilibrium at constant external pressure. Areas, or phase regions, are defined on these temperature-versus-composition plots within which either one or two phases exist. For an alloy of specified composition and at a known temperature, the phases present, their compositions, and relative amounts under equilibrium conditions may be determined.
Binary Systems without Solid Solution
Consider a system of two components, A and B, which are completely soluble in one another in the liquid state, but completely insoluble in one another in the solid state. The melting point of a liquid is normally depressed if the liquid contains some other substance in solution.
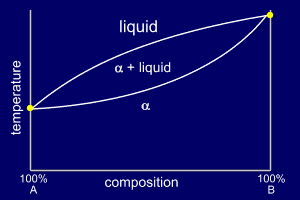
Binary Systems with Total Solid Solubility (Binary Isomorphous Systems)
It is possible for solids to form a solution. Solid solution means that the solute component enters and becomes a part of the crystalline solvent, without altering its basic structure. Solid solutions with complete solid solubility, i.e., solid solubility over the entire range of the composition, are possible to form. For a metallic binary solution to exhibit a complete solid solubility, for instance, both metals must have the same type of crystal structure, nearly identical atomic radii and electronegativities, and similar valences because it must be possible to replace all the atoms of the initial solvent with solute atoms without causing a change in crystal structure. The copper–nickel system displays this behavior. The copper–nickel system is termed isomorphous because of this complete liquid and solid solubility of the two components. The phase diagram shapes are as shown below:
Three different phase regions, or fields, appear on the diagram, an alpha (α) field, a liquid (L) field, and a two-phase field. The liquid L is a homogeneous liquid solution composed of both A and B. The α-phase is a substitutional solid solution consisting of both A and B atoms. At temperatures below about A and B are mutually soluble in each other in the solid state for all compositions.
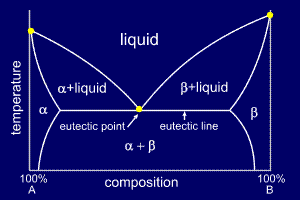
Binary Systems with Partial Solid Solubility (Binary Eutectic System)
In many cases, atom size, crystal structure or other factors restrict the ease with which solute atoms can be dissolved in the solvent in the solid state. Thus it is much more common to find that solids are partly soluble in one another rather than be either completely soluble or completely insoluble. The copper–silver system displays this behavior. The following is a binary system which shows partial solid solubility:
Three single-phase regions are found on the diagram: α, β, and liquid. The α-phase is a solid solution rich in A atom; it has B atom as the solute component. Otherwise in β-phase solid solution, A is the solute. Pure A and pure B are also considered to be α- and β-phases, respectively.
There are also three two-phase regions found for the system: The α+L, β+L and α+β. The α– and β-phase solid solutions coexist for all compositions and temperatures within the α+β phase field; the α+liquid and β++liquid phases also coexist in their respective phase regions.
References:
Callister, W.D. 2007. Materials science and engineering : an introduction 7ed
Lee, H.G. 2000. Chemical Thermodynamics for Metals And Materials
Before interpret and utilize phase diagram, it is necessary to understand component, system and phase. Components are pure metals and/or compounds of which an alloy is composed. For example, in a copper–zinc brass, the components are Cu and Zn. System may refer to a specific body of material under consideration (e.g., a ladle of molten steel). Or it may relate to the series of possible alloys consisting of the same components, but without regard to alloy composition (e.g., the iron–carbon system). Phase may be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics. Every pure material is considered to be a phase; so also is every solid, liquid, and gaseous solution.
Unary System
Perhaps the simplest and easiest type of phase diagram to understand is that for a one-component system. In a one-component system, or unary system, however, the composition does not vary, but must always be unity. Therefore there are only two variables which can vary: pressure and temperature. Every possible combination of temperature and pressure can be readily represented by points on a two-dimensional diagram. Three phases (solid-, liquid, and vapor-phase) are found on this type of phase diagram.
(www.teachersparadise.com)
Binary System
Another type of common phase diagram is that for two components or binary system. Binary phase diagrams represent the relationships between temperature and the compositions of phases at equilibrium at constant external pressure. Areas, or phase regions, are defined on these temperature-versus-composition plots within which either one or two phases exist. For an alloy of specified composition and at a known temperature, the phases present, their compositions, and relative amounts under equilibrium conditions may be determined.
Binary Systems without Solid Solution
Consider a system of two components, A and B, which are completely soluble in one another in the liquid state, but completely insoluble in one another in the solid state. The melting point of a liquid is normally depressed if the liquid contains some other substance in solution.
Binary Systems with Total Solid Solubility (Binary Isomorphous Systems)
It is possible for solids to form a solution. Solid solution means that the solute component enters and becomes a part of the crystalline solvent, without altering its basic structure. Solid solutions with complete solid solubility, i.e., solid solubility over the entire range of the composition, are possible to form. For a metallic binary solution to exhibit a complete solid solubility, for instance, both metals must have the same type of crystal structure, nearly identical atomic radii and electronegativities, and similar valences because it must be possible to replace all the atoms of the initial solvent with solute atoms without causing a change in crystal structure. The copper–nickel system displays this behavior. The copper–nickel system is termed isomorphous because of this complete liquid and solid solubility of the two components. The phase diagram shapes are as shown below:
(source: www.soton.ac.uk)
Three different phase regions, or fields, appear on the diagram, an alpha (α) field, a liquid (L) field, and a two-phase field. The liquid L is a homogeneous liquid solution composed of both A and B. The α-phase is a substitutional solid solution consisting of both A and B atoms. At temperatures below about A and B are mutually soluble in each other in the solid state for all compositions.
Binary Systems with Partial Solid Solubility (Binary Eutectic System)
In many cases, atom size, crystal structure or other factors restrict the ease with which solute atoms can be dissolved in the solvent in the solid state. Thus it is much more common to find that solids are partly soluble in one another rather than be either completely soluble or completely insoluble. The copper–silver system displays this behavior. The following is a binary system which shows partial solid solubility:
(source: www.soton.ac.uk)
Three single-phase regions are found on the diagram: α, β, and liquid. The α-phase is a solid solution rich in A atom; it has B atom as the solute component. Otherwise in β-phase solid solution, A is the solute. Pure A and pure B are also considered to be α- and β-phases, respectively.
There are also three two-phase regions found for the system: The α+L, β+L and α+β. The α– and β-phase solid solutions coexist for all compositions and temperatures within the α+β phase field; the α+liquid and β++liquid phases also coexist in their respective phase regions.
References:
Callister, W.D. 2007. Materials science and engineering : an introduction 7ed
Lee, H.G. 2000. Chemical Thermodynamics for Metals And Materials
Monday, November 1, 2010
Bioceramics And Their Applications
1. Hydroxyapatite (HA)
1.1. Introduction
1.3. Properties
1.3.3.1. In vitro cell response
1.4.1. HA as abrasive
2. Tricalcium Phosphate (TCP)
2.1. Introduction
2.3.1. Physicochemical properties
Bioceramics (calcium phosphates, bioactive glass) do not usually have osteoinductive property. However, several reports indicated osteoinductive properties of some calcium phosphate bioceramics such as those reported for coralline HA (derived from coral) or observed in some studies using BCP.
2.4. Applications
2.4.1. Applications in dentistry
Micromacroporous biphasic calcium phosphate bioceramics are largely used in orthopedics and effi cacy has been demonstrated in numerous preclinical and clinical studies, for example using specific shaped blocks (custom-designed) for spine arthrodesis (cage insert) and wedges for tibial valgization osteotomy of valgization.
3. Alumina (Al2O3)
3.1. Introduction
3.2.1. Physical properties
4. Zirconia (ZrO2)
4.1. Introduction
4.3.1. Physical Properties
5. Reference:
1) Kokubo, Tadashi. Bioceramics and their clinical applications. England : Woodhead Publishing.
2) Park, Joon. 2008. Bioceramics - Properties, Characterizations, and Applications. USA : Springer.
1.1. Introduction
Hydroxyapatite (HA) is a member of the apatite group of ceramics. The term “apatite” is derived from the Greek apatê, which means deceit or deception. It was called such for its diversity of form and color.
1.2. SourceThere are two sources of apatite: one biological and the other from mineral deposits, such as phosphate rock or phosphorite, a sedimentary rock the essential mineral components of which is carbonate fluoroapatite. Bone and teeth contain a HA-like mineral component that supports the majority of load in vivo. Deorganized bone and some sea corals (porites) are used to make implants.
1.3. Properties
The (bio)chemical and mechanical properties of HA are similar to those of bone and teeth. Their molecular structures are also similar, although the exact nature of the composite, the minerals and proteins, and their interactions are not fully understood.
1.3.1. Mechanical PropertiesThere is a wide variation in the reported mechanical properties of HA. Jarcho2)reported that fully densified polycrystalline specimens of HA synthesized by themhad average compressive and tensile strengths of 917 and 196 MPa, respectively.Kato2) noted a compressive strength of 3000 kg/cm2 (294 MPa), a bending strength of1500 kg/cm2 (147 MPa), and a Vickers hardness of 350 kg/mm2 (3.43 GPa).
1.3.2. Chemical PropertiesHydroxyapatite is considered bioactive, indicating that the ceramic may undergo ionization in vivo and that the rate of dissolution may depend on many factors — includeing degree of crystallinity, crystallite size, processing condition (temperature,pressure, and partial water pressure), and porosity. Hydroxyapatite is soluble in anacidic solution while insoluble in an alkaline one and slightly soluble in distilled water. The solubility of sintered HA is very low. The rate of solubility is 0.1 mg/yearin subcutaneous tissue. Hydroxyapatite reacts actively with proteins, lipids, and otherinorganic and organic species.
1.3.3. Biological properties 1.3.3.1. In vitro cell response
Substituted HA or HAP showed the following cell response: (a) carbonate-substituted apatite stimulate greater activity of osteoclasts compared with carbonate-free HAP or FAP or F-treated dentin (b) stimulation of proliferation and phenotypic expression of F-containing HAP or Ftreated bovine bone (c) difference in response between odontoblasts and osteoblasts
1.3.3.2. Tissue responseHA have bioactivity proerties, ability of the material to directly ‘bond’ to bone through chemical interaction and not physical or mechanical attachment. HA also have osteoconductive properties – an ability to serve as a scaffold or template to guide the newly forming bone along its surfaces.
1.4. Applications1.4.1. HA as abrasive
HA or apatitic abrasive (biphasic calcium phosphate) has gained popularity as the abrasive of choice for orthopedic and dental implant. Implant surface gritblasted with HA or apatitic abrasive was shown to be cleaner (free of inclusions) compared with alumina [31] and appear to promote higher bone contact.
1.4.2. Bone graft materials and scaffoldsDental applications of HA materials include: implants as immediate tooth root replacement, alveolar ridge augmentation , pulp capping , periodontal defects, bone regeneration with guided tissue regeneration membrane ; alveolar distraction osteogenesis, peri-implantitis defects, reconstruction of severely atrophic human maxillae and sinus lifts. Medical applications include: repair of bone defects, chin augmentation, ear implant by itself, or as a composite with high molecular weight polyethylene, spine cage, tibial osteotomy in patients with osteoarthritis, and as a percutanous device. HA or HAP is also used as component of calcium phosphate cement, CPC .
1.4.3. Implant coatingsIn spite of the many good qualities of HA and related calcium phosphates (e.g. B-TCP) such as bioactivity and osteoconductivity, they cannot be used in load-bearing areas because of their low fracture strength. On the other hand, metal implants, primarily titanium (Ti) or Ti alloy, are not bioactive and therefore do not bond directly to bone.
1.4.4. Drug delivery and other applicationsHA ceramic is used as gene carrier or transfection agents , for drug delivery such as delivery of anticancer drugs or bisphosphonate, or as scaffolds for bone regeneration by tissue engineering.
2. Tricalcium Phosphate (TCP)
2.1. Introduction
The term biphasic calcium phosphate (BCP) was first used by Ellinger et al. to describe the bioceramic previously described as ‘tricalcium phosphate’ but was shown by LeGeros in 1986 using X-ray diffraction (XRD) to consist of a mixture of 80% HA and 20% B-TCP.
2.2. FabricationBiphasic calcium phosphate (BCP), or intimate mixtures of HA and B-TCP, is obtained when synthetic calcium-deficient apatites (CDAs) are sintered above 900 °C [11,26,29] according to the following reaction:
2.3. Properties2.3.1. Physicochemical properties
Since B-TCP has a higher solubility than HA, the extent of dissolution of BCP ceramic of comparable macroporosity and particle size will depend on the HA/B-TCP ratio: the higher the ratio, the lower the extent of dissolution. This phenomenon may be caused by processing variables (sintering time and temperature).
2.3.2. Mechanical propertiesBCP ceramic prepared from a single calcium-deficient apatite phase was reported to exhibit higher compressive strength (2–12 MPa) compared with BCP ceramic prepared by mixing two unsintered calcium phosphate preparations (2 MPa): one that after sintering at 1200 °C resulted in only HA and the other that resulted in only B-TCP [48]. The initial mechanical property is not the best criterion for efficacy of bone ingrowth.
2.3.3. Bioactivity and osteogenic propertiesBioceramics (calcium phosphates, bioactive glass) do not usually have osteoinductive property. However, several reports indicated osteoinductive properties of some calcium phosphate bioceramics such as those reported for coralline HA (derived from coral) or observed in some studies using BCP.
2.4. Applications
2.4.1. Applications in dentistry
Dental applications of BCP include prevention of bone loss after tooth extraction, repair of periodontal defects and sinus lift augmentation.
2.4.2. Applications in orthopedicsMicromacroporous biphasic calcium phosphate bioceramics are largely used in orthopedics and effi cacy has been demonstrated in numerous preclinical and clinical studies, for example using specific shaped blocks (custom-designed) for spine arthrodesis (cage insert) and wedges for tibial valgization osteotomy of valgization.
3. Alumina (Al2O3)
3.1. Introduction
Aluminium oxide (Al2O3), more commonly known as alumina, is the most widely used oxide ceramic material. Bauxite (hydrated aluminum oxide) and native corundum (aluminum oxide mineral) are the main sources of high-purity alumina. As a raw material, Al2O3 powder is produced in large quantities from the mineral bauxite, by the Bayer process. Bayer process, which yields D-alumina. The Bayer process involves dissolution of crushed bauxite in sodium hydroxide (NaOH) solution under pressure at high temperatures (up to 300ºC) to form a supersaturated sodium aluminate solution.
3.2. Properties3.2.1. Physical properties
Additives or impurities determine the colour of alumina, in addition to the sintering atmosphere, and by the interaction with ionising radiation. Alumina is generally white but can sometimes be pink (88% alumina) or brown (96% alumina). When chromium oxide (Cr2O3) is added, it reacts with Al2O3 to form a solid solution. The amount of chromium oxide added will determine whether the colour of alumina changes to pink or ruby. When medical-grade alumina is sintered in air together with the addition of magnesia, it will appear as ivory. Alumina turns white when it is sintered in reducing atmosphere or if it contains traces of silica.
3.2.2. Mechanical propertiesBecause of their strong bonding, alumina ceramics have very high melting or, more appropriately, dissociation temperatures, hence the production of alumina ceramics can only be achieved with high-temperature sintering. During the sintering process, powders are heated usually to two-thirds of their melting temperature. As shown earlier, during this densification particles bond together to form necks between the particles, which subsequently reduce the surface area and cause the powder to consolidate.
3.3. ApplicationsHigh-purity alumina bioceramics have been developed as an alternative to surgical metal alloys for total hip prosthesis and tooth implants. Their high hardness, low friction coefficient and the excellent corrosion resistance of alumina offer a very low wear rate at the articulating surfaces in orthopaedic applications. Alumina has the ability to be polished to a high surface finish. Other applications for alumina in orthopaedic and maxillofacial applications include porous coatings for femoral stems, alumina spacers employed specifically in revision surgery (Huckstep and Sherry, 1996), knee prostheses (see Fig. 10.4), and in the past as polycrystalline and single crystal forms in dental applications as tooth implants.
4. Zirconia (ZrO2)
4.1. Introduction
Zirconium oxides (zirconia) have been used for the purpose of fabricating implants. Some are called “fake diamond” or “cubic zirconia” since some zirconia single crystals can be of gem grade and made into jewels. Some of their mechanical properties are as good or better than those of alumina ceramics. They are highly biocompatible, like other ceramics, and can be made into such large implants as the femoral head of a hip joint replacement. Some of their drawbacks include the fact that they exhibit high density, low hardness, and phase transformations under stress in aqueous conditions, thus degrading their mechanical properties.
4.2. SourceZircon (ZrSiO4) is the most commercially important zirconium mineral and is found mostly in the mineral baddeleyite. Zircon is a gold-colored silicate of zirconium, a mineral found in igneous and sedimentary rock and occurring in tetragonal crystals colored of many colors. The transparent varieties are usually deposited in beach sand, and are used as gems. Zircon is first chlorinated to form ZrCl4 in a fluidized bed reactor in the presence of petroleum coke. A second chlorination is required for highquality zirconium. Zirconium is precipitated with either hydroxides or sulfates, and then calcined to its oxide.
4.3. Properties4.3.1. Physical Properties
Zirconia undergoes an allotropic phase transition from monoclinic to tetragonal at 1000~1200ºC, and from tetragonal to cubic at 2370ºC. The cubic-to-monoclinic and tetragonal
phase transition is diffusionless and accompanies a volume expansion of about 7%. The cubic structure of zirconia belongs to the group of fluorite (CaF2) structures.
4.3.2. Mechanical propertiesThe strength of the partially stabilized zirconia with yttrium oxide (Y–TZP) showed the highest flexural strength and fracture toughness. However, the Weibull modulus was lower than the yttrium magnesium oxide-stabilized zirconia (Y–Mg–PSZ). It is also interesting that the increased fracture toughness is due to a phase transformation caused by cessation of crack propagation.
4.4. ApplicationsYttrium-stabilized zirconia has been used to fabricate the femoral head of total hip joint prostheses and has two advantages over alumina. One is the finer grain size and well-controlled microstructure with minimum residual porosity, resulting in a better tribological material than with alumina. The other is higher fracture strength and toughness due to the phase transformation toughening process. Approximately 20% of the prosthetic femoral heads manufactured in the world are made of ceramic, with a strongly growing market (i.e. more than 25% growth for the alumina–alumina coupling between 2002 and 2004). Up to the year 2000,approximately 40% of ceramic heads were zirconia and the remaining alumina.
5. Reference:
1) Kokubo, Tadashi. Bioceramics and their clinical applications. England : Woodhead Publishing.
2) Park, Joon. 2008. Bioceramics - Properties, Characterizations, and Applications. USA : Springer.
Solder Bebas Timbal
Solder merupakan paduan logam yang digunakan untuk menggabungkan dua atau lebih komponen. Komponen yang disatukan itu bisa berupa logam atau paduan logam lain. Solder digunakan secara ekstensif pada industri elektronik untuk menyatukan komponen secara fisik agar dapat menghantarkan sinyal listrik.
Solder bekerja dengan meleleh oleh adanya panas. Lelehan solder kemudian mengalir dan membuat kontak dengan komponen yang akan bergabung (yang tidak meleleh). Setelah pembekuan, solder membentuk ikatan fisik dengan semua komponen.
Sebelumnya, solder terbuat dari paduan Pb-Sn. Paduan ini murah dan mempunyai titik leleh rendah sehingga memungkinkan bekerja pada suhu dibawah titik leleh komponen yang akan disatukan. Solder Pb-Sn mempunyai komposisi 63 wt% Sn-37 wt % Pb. Menurut diagram fasa Pb-Sn, komposisi ini dekat dengan titik eutektik dan mempunyai temperatur leleh sekitar 183 oC. Temperatur ini adalah temperatur paling rendah yang bisa dicapai dengan keberadaan fasa liquid. Paduan ini kemudian dikenal sebagai solder Pb-Sn eutektik.
Namun sayangnya, timbal merupakan logam toksik dan mempunyai dampak lingkungan yang serius apabila lepas ke air tanah dari landfill atau ke udara jika masuk ke incinerator. Akibatnya, beberapa negara telah membatasi penggunaan timbal ini. Hal ini mendorong pengembangan solder bebas timbal yang masih mempunyai temperatur leleh rendah. Beberapa diantaranya adalah paduan terner (tersusun atas tiga logam) solder timah-perak-tembaga dan timah-perak-bismut. Komposisi beberapa solder bebas timbal disajikan pada tabel berikut.
Ada beberapa kesulitan menggunakan solder bebas timbal. Pertama adalah rendahnya rendahnya kemampuan alir dari solder sehingga kemampuan solder untuk menyatukan komponen secara elektrik berkurang. Kemampuan alir ini diketahui tidak dapat ditingkatkan dengan peningkatan temperatur. Kesulitan kedua adalah tingginya titik leleh. Secara umum titik leleh solder bebas timbal adalah 20 hingga 45 oC lebih tinggi daripada solder konvensional. Akibat dari temperatur tinggi adalah berkurangnya umur tip solder karena oksidasi atau erosi, karbonasi pada solder, dan percikan api. Penggunakan solder bebas timbal dikatakan dapat mengurangi umur tip hingga 4 kali.
Solder bekerja dengan meleleh oleh adanya panas. Lelehan solder kemudian mengalir dan membuat kontak dengan komponen yang akan bergabung (yang tidak meleleh). Setelah pembekuan, solder membentuk ikatan fisik dengan semua komponen.
Sebelumnya, solder terbuat dari paduan Pb-Sn. Paduan ini murah dan mempunyai titik leleh rendah sehingga memungkinkan bekerja pada suhu dibawah titik leleh komponen yang akan disatukan. Solder Pb-Sn mempunyai komposisi 63 wt% Sn-37 wt % Pb. Menurut diagram fasa Pb-Sn, komposisi ini dekat dengan titik eutektik dan mempunyai temperatur leleh sekitar 183 oC. Temperatur ini adalah temperatur paling rendah yang bisa dicapai dengan keberadaan fasa liquid. Paduan ini kemudian dikenal sebagai solder Pb-Sn eutektik.
Namun sayangnya, timbal merupakan logam toksik dan mempunyai dampak lingkungan yang serius apabila lepas ke air tanah dari landfill atau ke udara jika masuk ke incinerator. Akibatnya, beberapa negara telah membatasi penggunaan timbal ini. Hal ini mendorong pengembangan solder bebas timbal yang masih mempunyai temperatur leleh rendah. Beberapa diantaranya adalah paduan terner (tersusun atas tiga logam) solder timah-perak-tembaga dan timah-perak-bismut. Komposisi beberapa solder bebas timbal disajikan pada tabel berikut.
Ada beberapa kesulitan menggunakan solder bebas timbal. Pertama adalah rendahnya rendahnya kemampuan alir dari solder sehingga kemampuan solder untuk menyatukan komponen secara elektrik berkurang. Kemampuan alir ini diketahui tidak dapat ditingkatkan dengan peningkatan temperatur. Kesulitan kedua adalah tingginya titik leleh. Secara umum titik leleh solder bebas timbal adalah 20 hingga 45 oC lebih tinggi daripada solder konvensional. Akibat dari temperatur tinggi adalah berkurangnya umur tip solder karena oksidasi atau erosi, karbonasi pada solder, dan percikan api. Penggunakan solder bebas timbal dikatakan dapat mengurangi umur tip hingga 4 kali.
Labels:
pernak pernik
Subscribe to:
Posts (Atom)